If a solid substance is added to a liquid, the solid may dissolve
forming a solution.
Liquids and gases may also dissolve in liquids. In that situation, the
liquid is a solvent while the dissolving substance is called a solute.
Sometimes, solids and liquids may not dissolve in a given liquid. The
substances are said to be insoluble. The combination is called a mixture. The
constituents of the mixtures can be separated by physical means. Particular
methods are used in the separation depending on the properties of the
constituents. Methods of separating mixtures into their components are important
because pure substances are needed for industrial processes.
Separation techniques
Chemists have developed many different methods of separation of mixtures from simple or complex compounds. Methods used depend on what is in the mixture and properties of the substances present. Also depends on whether the substances to be separated are solid, liquid or gas.
Some methods of separating mixtures are:
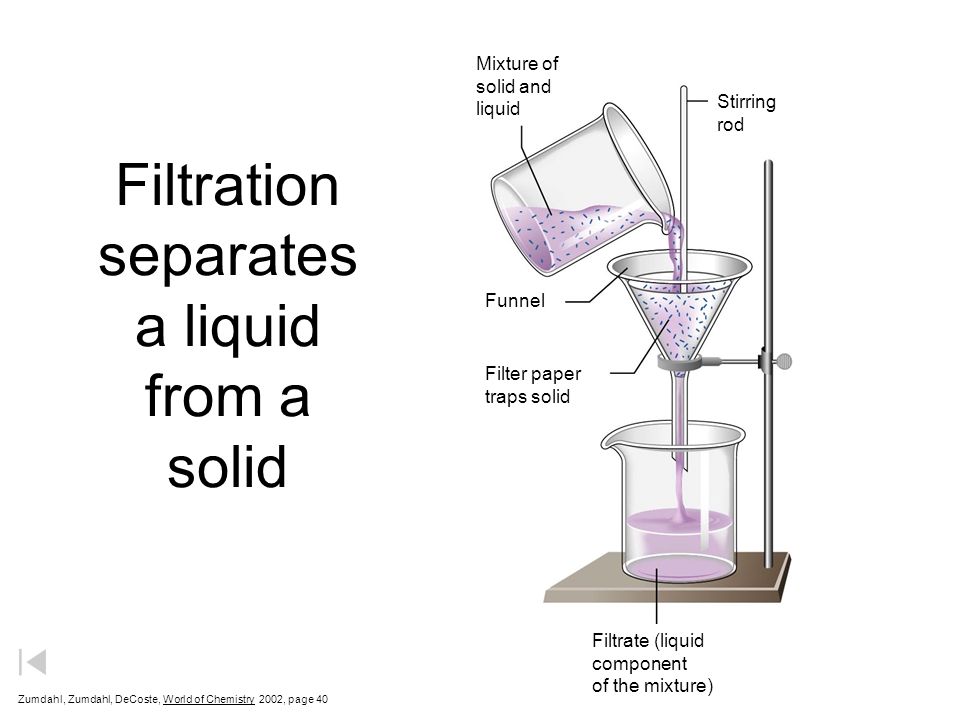
Filtration separates
a solid from a liquid using a filter paper supported in a funnel. Decanting
also separates a solid from a liquid. However, in decanting, the mixture is
left to settle. The liquid is then carefully poured off the solid.
A centrifuge separates a
solid and a liquid by spinning a tube containing the mixture in a circle at
several thousands of revolutions per minute. The solid is forced to the bottom
of the tube allowing the liquid to be decanted easily.
Simple
distillation is similar to fractional. The process involves the
evaporation of a liquid from a mixture of a liquid and a solid followed by
condensation of the vapour. The condensed liquid is called the distillate. For
example sea water on distillation will produce pure water as the distillate and
sea salt as a residue.
v. Fractional distillation separates mixtures of liquids
(fraction) into their components. Boiling the mixtures causes the components to
vaporise in succession - the liquid with the lowest boiling point vaporising
first. The vapours are condensed in a water-cooled condenser and collected as
distillates. For example, wine on distillation will produce two distillates,
ethanol (alcohol) and water, in that order.
vi. Crystallisations occur when a hot, saturated solution is
allowed to cool. The crystals that form will be the pure solid component if
solvent is the only other component. Decanting the remaining solution allows
the crystals to be dried on a tissue and so obtained pure.
vii. Paper chromatography is the process of separating a mixture of
solids, in solution, on a sheet of paper.
The table below summarises the types of separation techniques used in
industry and in the laboratories.
|
Substance |
Deposit |
Result |
Means of Separation |
|
Water |
Sodium Chloride |
Solution |
Evaporation |
|
Water |
Copper Sulphate |
Saturated Solution |
Crystallization |
|
Water |
Soil |
Mixture |
Filtration |
|
Sulphur |
Iron |
Mixture |
Magnetization |
|
Iodine |
Sodium Chloride |
Mixture |
Sublimation |
|
Common Salt |
Water |
Solution |
Distillation |
|
Water |
Alcohol |
Solution |
Fractional Distillation |
|
Paraffin |
Water |
Mixture |
Decanting |
|
Colour |
Colour |
Solution |
Chromatography |
|
Milk |
Butter |
Mixture |
Centrifugation |
When the substances are separated, the final products approach the
original purity of the original substance.
Substances
are made up of some basic substances called “elements”. Each element is composed of its own kind of particles
called “atoms”. For example, aluminium is an element which is made up of only
aluminium atoms. An Element cannot be
broken any further. Elements can be classified as “metals and nonmetals”.
The atoms
of all elements are extremely small to be seen. The smallest atom known is
hydrogen with diameter 7x10-8mm. atoms of different elements have
different diameters as well as different masses.
Chemist use shorthand symbols to label the elements and their atoms. The
symbol consists of one, two or three letters, the first of which must be a capital.
For example, C is used for Carbon, Ca for Calcium and Cl for Chlorine. Some
symbols seem to have no relationship to the name of the element, for example Na
for Sodium and Pb for lead. These symbols came from their Latin names Natrium
for Sodium and Plumbum for Lead.
To make sense of the material world around us, we need methods for
physically separating the many and varied mixtures that we come across. Being
able to purify and identify the many substances present in these mixtures not
only satisfies our curiosity but is crucial to our well-being and health. A
range of physical techniques are available to make the necessary separations. They all depend in some way on a difference in the physical
properties of the substances in the mixture.
The most useful separation method for a particular mixture depends on:
•
the type of mixture, and
•
which substance in the mixture we are most interested
in.
Separating heterogeneous mixtures
In some ways these are the easier mixtures to separate. Quite often,
just leaving them to stand helps with the separation. This is often the first
stage in separating mixtures of immiscible liquids. It is also often used to
separate solid-in-liquid suspensions if the particles of solid are large
enough. Once the solid has settled to the bottom (sedimented), the liquid can
be carefully poured off (this is called decantation).
A more generally useful method than decantation for separating solids
from liquids is filtration. Here the insoluble material is collected as a residue on filter paper.
Filtration is useful because both phases can be obtained in one process. The
liquid phase is collected as the filtrate
The process can be speeded up by using a vacuum pump to ‘suck’ the liquid
through the filter paper in a Buchner funnel and flask.
Various large-scale filtration methods are used in industry. Perhaps the
most useful of these are the filter-beds to purify water for household use.
Table 2.5 Methods of separating substances from mixtures
|
Type of
mixture |
Mixture |
Method of
separation |
|
Heterogeneous |
Solid + solid (powdered mixture) |
use some difference in properties, e.g. density,
solubility, sublimation, magnetism |
|
Suspension of solid in liquid |
filtration or centrifugation |
|
|
Liquid + liquid (immiscible) |
use a separating funnel or decantation |
|
|
Homogeneous |
Solution of solid in liquid |
to obtain solid: use evaporation (crystallisation)
to obtain liquid: use distillation |
|
Two (or more) liquids mixed together (miscible) |
fractional distillation |
|
|
Solution of two (or more) solids in a liquid |
chromatography |
Another means of separating an insoluble solid from a liquid is centrifugation where the mixture is
spun at high speed in a centrifuge. Here, it is no longer the force of gravity
on the solid particles that causes settling. Instead, there is a huge
centrifugal force acting on the particles due to the high-speed spinning of the
samples. This causes the solid to be sedimented at the bottom of the centrifuge
tube. The liquid can be decanted off carefully.
Mixtures of two immiscible liquids can be separated if the mixture is
placed in a separating funnel and allowed to stand. The liquids separate
into different layers. The lower, denser layer is then ‘tapped’ off at the
bottom. This type of separation is useful in industry. For
example, at the base of the blast furnace the molten slag forms a separate
layer on top of the liquid iron. The two can then be ‘tapped’ off separately.
The method is also very useful in organic chemistry as part of a process called
‘solvent extraction’.
The separation of a solid from a mixture of solids depends largely on
the particular substance being purified. Some suitable difference in physical
properties needs to be found. Usually it helps if the mixture is ground to a
powder before any separation is attempted.
1.
Separations
based on differences in density
‘Panning’ for gold is still carried out in the search for new deposits.
In Amazonia, river-beds are mechanically sifted (‘vacuum-cleaned’) to collect
gold dust. These methods depend on the gold dust being denser than the other
substances in the river sediment. This type of method is also used in purifying
the ores of zinc and copper, although in these cases the metals are less dense
than the ores and so float on the surface.
1.
The mixture of immiscible liquids settles into two
layers, as the liquids do not mix
2.
The tap is opened to let only the bottom layer run
info the beaker
3.
The tap is closed and the beaker is changed. The tap
is opened to let the top layer run out
 |
| A separating funnel can be used to separate two immiscible liquids. |
2.
Separations
based on magnetic properties
Magnetic iron ore can be
separated from other material in the crushed ore by using an electromagnet. In
the Amazonian gold diggings, magnets are used to clean away iron-containing,
red-brown dust from the powdered gold. In the environmentally and economically
important processes of recycling metals, iron objects can be picked out from
other scrap metal using electromagnets.
3. Separations based on differences in solubility
One very useful way of separating a soluble substance from a solid mixture is as follows. The mixture is first ground to a powder. A suitable liquid solvent is added. The solvent must dissolve one of the solid substances present, but not the others. The solvent is often water, but other liquids can be useful. The mixture in the solvent is then warmed and stirred. Care must be taken at the warming stage when using solvents other than water.
The warm mixture is then filtered. This gives the insoluble substances as a residue on the filter paper, which can be dried. The soluble substance is in the liquid filtrate. Dry crystals can be obtained by evaporation and crystallisation.
The gold prospectors in Brazil and Zimbabwe still use an immensely dangerous version of this method to extract the gold from other substances. The solvent they use is mercury, which dissolves the gold. The gold is then recovered from solution by evaporating off the mercury with a blowtorch.
The unwanted residues, contaminated with mercury, are thrown into the rivers. Damage to the environment from this activity is very likely because mercury is poisonous to living things.
4. Separations based on sublimation
A solid that sublimes can be separated from others using this property.
Separating homogeneous mixtures
The separation of homogeneous mixtures is often slightly more
complicated because there is no physical separation of the phases in the
original mixture. The methods of separation usually depend on solubility
properties or on differences in boiling point.
| Separation of Ammonium chloride |
Separating a solid from solution in a liquid can be carried out by evaporation
or crystallisation. Evaporation gives only a powder, but
crystallisation can result in proper crystals. Both processes begin by
evaporating away the liquid but, when crystals are needed, evaporation is
stopped when the solution has been concentrated enough. Figure 2.10 shows how
this can be judged and done safely. The concentrated solution is allowed to
cool slowly. The crystals formed can then be filtered off and dried.
Separating a liquid from a solution is usually carried out by
distillation. The boiling point of the liquid is usually very
much lower than that of the dissolved solid. The liquid can easily be
evaporated off in a distillation flask. It is condensed by passing it down a
water-cooled condenser, and then collected as the distillate.
 |
| Separation by distillation - An evaporation method |

While the solvent is evaporating, dip a glass rod into the solution from time to time. When small crystals form on the rod, take the solution off the water bath and leave it to cool.
This method should not be used if the solvent is flammable. Instead, use an electrical heating element and an oil or water bath.
Separating the liquids from a mixture of two (or more) miscible liquids
is again based on the fact that the liquids will have different boiling points.
However, the boiling points are closer together than for a solid-in-liquid
solution and fractional distillation must be used.
For example, ethanol boils at 78 °C whereas water boils at 100°C. When
the mixture is heated, ethanol and water vapours enter the fractionating
column. Glass beads in the column provide a large surface area for
condensation. Evaporation and condensation take place many times as the vapours
rise up the column. Ethanol passes through the condenser first as the
temperature of the column is raised above its boiling point. Water condenses in
the column and flows back into the flask because the temperature of the column
is below its b.p. of 100°C.
 |
| Separating a mixture of ethanol (alcohol) and water by fractional distillation |
The temperature on the thermometer stays at 78 °C until all the ethanol
has distilled over. Only then does the temperature on the thermometer rise to
100°C and the water distils over. By watching the temperature carefully, the
two liquids (fractions) can be collected separately.
Fractional
distillation is used to separate any solution containing liquids
with different boiling points. It can be adapted as a continuous process and is
used industrially to separate:
•
the various fractions from crude oil,
•
the different gases from liquid air .
Solubility
The solubility of solids in liquids
Probably the most important and common examples of mixtures are
solutions of solids in liquids.
Such a solution is made up of two parts:
•
the solid that dissolves is known as the solute,
•
the liquid in which it dissolves is called the
solvent.












0 Comments