Gases in the atmosphere are held in an envelope around the earth by gravity. The atmosphere is 80 km thick and is divided into four layers, namely troposphere, stratosphere, mesosphere, and thermosphere. About 75% of mass is found in the layer nearest to earth, the troposphere.
 |
| Components of the Atmospheric Air |
Atmospheric air definition
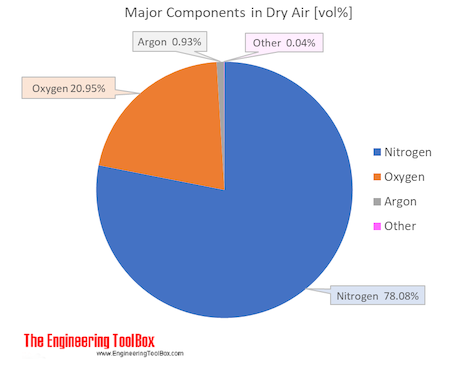
Atmospheric air is the type of air that can be observed under atmospheric conditions. Air is a mixture. When a sample of dry, unpolluted air is taken from the
atmosphere and analysed, the composition by volume is often found to be:
|
Component |
% |
Boiling
Point °C |
Melting
Point °C |
|
Nitrogen |
78.08 |
-196 |
-210 |
|
Oxygen |
20.95 |
-183 |
-218.4 |
|
Argon |
0.93 |
-186 |
-189.3 |
|
Carbon dioxide |
0.03 |
|
|
|
Neon |
0.002 |
-246.1 |
-249 |
|
Helium |
0.0005 |
-269 |
-272 |
|
Krypton |
0.0001 |
-153 |
-157.36 |
|
Xenon and minute other gases |
0.0001 |
-108 |
-111.9 |
Carbon dioxide is present in the air in proportion of 0.03% by volume.
It is formed during the combustion of all common fuels (wood, coal, coke,
natural water gas, petrol, paraffin) all of which contain carbon.
C(S) + O2 (g) →CO2 (g)
The presence of carbon dioxide in the air is detected through aspirating
air through a boiling tube containing calcium hydroxide solution. After some
time, the solution goes milky showing the presence of carbon dioxide.
All animals breathe out carbon dioxide as waste product. The proportion
of carbon dioxide remains constant although it is produced in large quantities
through these means.
FRACTIONAL DISTILLATION OF LIQUID AIR
• The air is passed through fine filters to remove dust.
• The air is cooled to about -80 degrees Celsius to remove water
vapour because it can result into serious blockage of pipes.
• The cold air is compressed to about 100 atmospheres of pressure.
This warms up the air-so it is passed into a heat exchanger to cool it down
again.
• The cold, compressed air is allowed to expand rapidly and that
cools it still further.
• Process of compression followed by expansion is repeated until
the air reaches a temperature below -200 degrees Celsius. At this temperature
most of the air liquefies.
The gases are collected at their different boiling
points. Refer to the table above.
OXYGEN
MEASURING THE PERCENTANGE OF OXYGEN IN THE AIR
Consider 100 cm3 in a syringe passed back and forth over
heated copper granules as shown on the diagram below.
2KClO3 (s) → 2KC1(S) + 3O2(g)
Since oxygen is almost the same density as air, it
cannot be collected by displacement of air. The gas is collected over water.
From Hydrogen Peroxide (H2O2)
Hydrogen Peroxide (H2O2) is added drop by drop to Manganese (IV) Oxide
which catalyses decomposition.
2H2O2(aq) → 2H2O(aq)
+ O2(g)
Oxygen is collected over water. Passing the gas over anhydrous calcium
chloride you III may collect dry oxygen.
From Hydrogen Peroxide (H2O2) and Potassium Manganate (VII)
Hydrogen Peroxide (H2O2) is added drop by drop to Potassium
Manganate (VII) in the presence of dilute sulphuric acid. Oxygen is liberated
until all the Manganate (VII) is decomposed. The mixture becomes colourless.
H2O2(aq)+2 KMnO4 (aq) + 3H2SO4(aq)
→K2SO4(aq) + 2MnSO4 (aq)
+ 8H2O(l) + 5O2 (g)
Oxygen is collected over water. Passing the gas over anhydrous calcium
chloride you may collect dry oxygen.
PROPERTIES OF OXYGEN
·
Colourless
·
Odourless,
·
Neutral
·
Slightly soluble in water
·
Has approximately the same density as air
·
Reacts with metals and non-metals to form basic and
acidic oxides.
USES OF OXYGEN
The uses of oxygen can be classified as medical, industrial,
recreational and for research purposes. The uses of oxygen are as follows:
•
Large quantities are used to convert pig iron into
steel and for producing very hot flames for welding by mixing with gases such
as ethylene (acetylene).
•
It is used in hospitals to help patients with
breathing difficulties.
•
People such as mountaineers and divers use oxygen.
•
It is carried in space rockets so that the hydrogen,
and kerosene fuel can bum.
•
Space shuttles use oxygen gas in fuel cells, which
convert chemical energy into electrical energy.
•
Astronauts must carry their own supply of oxygen as do
fire fighters.
•
It is used to restore life in polluted lakes and
rivers and in the treatment of sewage.
ACTION OF OXYGEN ON METALS AND NON-METALS
Action of oxygen with Metals
Oxygen reacts with metals to form basic oxides. It reacts with some
metals more readily than others. The oxides of K, Na and Ca readily react with
water to form a hydroxide.
CaO(S) +H2O(i)→Ca (OH)2(aq)
The hydroxides of K and Na are very soluble in water while Ca is sparingly
soluble. The solution turns litmus paper blue.
The oxides of K, Na, Ca, Mg, and A1 are not reduced by hydrogen. Oxides
of Hg, Ag and Au are decomposed when heated.
Action of oxygen with Non-metals
Oxygen reacts with non-metals to form acidic oxides.
S(s)+ O2 (g) → SO2(g);
SO2 (g)+
H20(i) →H2SO3 (aq)
(s)+ 5O2 (g) →P4O10 (S);
2NO(g) +O2 (g) →2NO2 (g);
CO2 (g)+ H2O(i) →H2 CO2(aq)
P4O10 (S)+ 6H2O(i)→4H3PO4 (aq)
2NO2 (g)+ H2O
(l)→HNO3 (aq) + HNO2 (aq)
Non-metal oxides dissolve in water to form acids. The acids turn the
litmus paper red.
CARBON DIOXIDE
Laboratory Preparation of Carbon dioxide
Carbon dioxide is produced by reacting carbonate with dilute acid.
Example
CaCO3(s) + 2HCl(aq) → CaCl2 (aq) + H2O(1)
+ CO2(g)
The gas is collected through the wafer in a gas jar. If dry gas is
required, it is passed through concentrated sulphuric acid and collected
through upward displacement of air.
Industrial Preparation of Carbon dioxide
Carbon dioxide is prepared by passing air through a thick layer of
white-hot coke in a producer. Strong heat is liberated in the process.
C(s) + O2(g)→ CO2(g)
As the gas rises, it reacts with white-hot coke. This absorbs a lot of
heat.
CO2 (g) CO2(g) + C(S) →2CO(g)
The resulting gas is a mixture of carbon monoxide and carbon dioxide.
The gas is mixed with air further to produce carbon dioxide.
2COg + O2(g) + Cg) → 2C02(g)
Properties of carbon dioxide
·
Colourless
•
Sparingly soluble in water to form weak carbonic acid
H2O (l) + CO2 (g)→ H2CO3
(l)
·
Denser than air
·
Extinguishes a lighted splint
•
Supports combustion for strong substances (e.g., Mg)
2 Mg(S) + CO2(g)→
2MgO(s) + C(S)
• Reacts with strong alkali to form carbonates and bicarbonates.
For example, Calcium Hydroxide to produce a milky stuff. The milkish is due to
a suspension of the insoluble substance, calcium carbonate.
CO2 (g) + Ca(OH)2(aq)
→ CaCO3(s) + H2O(1)
When bubbles through the liquid continue, it will eventually become
clear. This is because of the formation of the soluble calcium hydrogen
carbonate.
CaCO3(S) + H2O (l) + CO2(g)→
Ca(HCO3)2(aq)
It is significant to note that carbon dioxide reacts with strong alkalis
to form carbonates. Excess carbon dioxide results in hydrogen carbonate being
formed.
Na2CO3(aq) + HO(l) +
CO2(g) → 2Na(HCO3)(S)
Uses of Carbon dioxide
• Carbonated Drinks: Large quantities are used to
make soda, mineral water as well as beer. The carbon dioxide gas is bubbled
into the liquid under high pressure, which increases solubility.
• Fire extinguishers: It is used in fire
extinguishers for use in electrical fires. Carbon dioxide is denser than air
and forms a layer around the burning material. It covers the fire and starves
of oxygen. Carbon dioxide does not burn and so the fire is put out.
• Refrigerant: Solid carbon dioxide (dry ice) is used
for refrigerating ice cream, meat, soft fruits as it is colder than ice and it
sublimes.
• Special effects: Carbon dioxide is used to
create smoke effects in concerts and TVs. Dry ice is placed in boiling water
and it forms thick cloud of white smoke. It stays close to the floor due to its
density.










0 Comments